 Image via Wikipedia
Image via Wikipedia
In addition to contributing to a global greenhouse effect, some of the carbon dioxide from cars, factories and power plants dissolves in the ocean, creating the same carbonic acid that gives soda pop its tang. The process makes seawater slightly more acidic, and also gobbles up carbonate, a basic building block of seashells.The result can be an environment where shells dissolve, destroying plankton, marine snails and other small creatures that sustain the rest of the marine food web. Acidified water also can kill fish eggs and larvae.
Byrne and his colleagues developed a more precise way to measure pH, using a dye that turns from purple to bright yellow as acidity increases. On board the ship, they used instruments called spectrophotometers to measure the color change and nail pH levels 10 times more accurately than possible before.
Debby Ianson, an ocean climate modeler for Canada's Institute of Ocean Sciences who was not involved in the project, said the approach is a good one. "We need studies like this," she wrote in an e-mail.
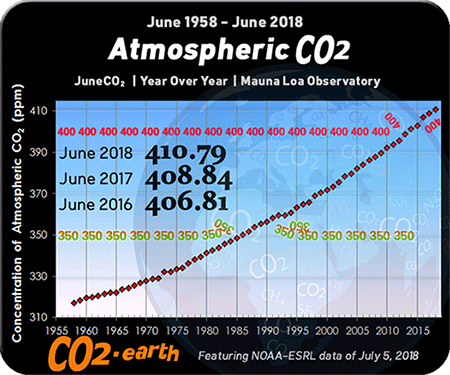
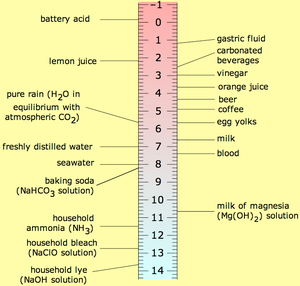
As expected, the researchers found acidification was strongest in the top layer of water, closest to the atmosphere. Normal seawater is slightly alkaline, with a pH value of about 8. Over the past 15 years, average pH levels in the top 300 feet of the ocean dropped 0.026 pH units. That sounds tiny, but is equivalent to a 6 percent jump in acidity, Byrne said.

![Reblog this post [with Zemanta]](http://img.zemanta.com/reblog_e.png?x-id=48da08d6-f1be-4533-8e21-88362b55b7de)